Document Type : Original Research Article
Authors
1 Department of Chemistry, Shahrood University of Technology, Shahrood, Iran
2 Department of Chemical Engineering, Babol Noshirvani University of Technology, Babol, Iran
Abstract
Rigid aromatic backbone in high-performance polymers such as Kevlar has been known to be effective in their remarkable properties. Four new polyureas were synthesized based on the 2, 3-Dihydro-1, 4-phthalazinedione with various aromatic and aliphatic diisocyanates to mimic the properties of high-performance polymers with accessible monomers, facile synthesis procedures, and more economic. The chemical structure of polyureas was validated using Infrared spectroscopy (IR) and elemental analysis. Molecular weight was determined using the Mark-Houwink equation. Weight losses of these polyureas with temperature were determined by thermal gravimetric analysis (TGA). Depending on the structure of polyureas, that had an average melting temperature of 153-290 °C, Solubility parameters were calculated using the group molar attraction constants of Hoy. Polymers were almost insoluble in different solvents, as demonstrated by attempts to solubilize in several solvents at 40 °C.
Graphical Abstract
Keywords
Main Subjects
Introduction
Polyureas are a class of engineering polymers with superior mechanical, hydrolatic stability, chemical resistance, and distinctive optical properties [1, 2]. This synthetic polymer is derived from the step-growth reaction between diamine and diisocyanate groups [3]. Owing to the capability of utilizing the diversity of monomers in polyureas synthesis, this polymer's chemical, mechanical features, and degradability is tailorable [4, 5]. Moreover, polyureas enjoy versatile post-processing into fibers, films, and foams [6]. Hence, a wide variety of applications, including coating, adhesive in the construction of biomedical implants, and sports equipment have been discussed for polyureas [3‒11].
Kevlar is a high-performance, waterproof, anti-static, flame retardant, heat resistant, and high strength polymer with vast professional applications such as aerospace engineering, body armor, bulletproof vests, and car brakes [12, 13]. One crucial challenge with Kevlar is its high costs [14]. Considering the unbeatable properties of Kevlar brings it to mind to add more aromatic structures into the backbone of polymers to make novel polymers with unique properties, though cheaper than Kevlar.
Phtalhydrazide and tetrabromo hydrazide are two nucleophilic aromatic compounds extensively synthesized for organic and pharmaceutical compounds [15, 16]. The ease of preparation of these aromatic compounds and their tendency to react with bifunctional monomers, make them an excellent candidate for step-growth polymerization.
With their aforementioned susceptibilities and their biofucntional nature of synthesizing, polyureas are excellent candidates for assessing their novel structure and properties after entering aromatic structures in their backbone. In line with our precedent studies [17, 18], four polyureas based on 2, 3-Dihydro-1, 4-phthalazinedione were synthesized in this research, and their chemical structure, polymer molecular weight, and thermal properties were evaluated. The theoretical solubilities of synthesized polyureas were calculated in different solvents and then analyzed experimentally.
Experimental
Materials and methods
All chemicals were purchased from Merck and Aldrich chemical companies and used without further purification.
Monomer synthesis
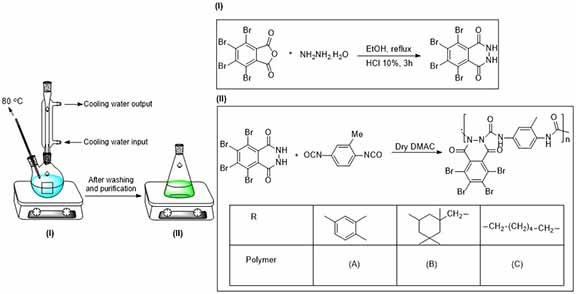
To prepare 2, 3-dihydro-1, 4-phthalazinedione, the mixture of phthalic anhydride (0,0146 mol) and hydrazine hydrate (0.032 mol) was reflux in ethanol at 80 °C for 90 min. Then the reflux was followed by adding a solution of HCl (10 wt%, 1 mL) for another 90 min at the same temperature. The obtained white precipitate was first washed with water, then stirred in ethanol for an hour.
5, 6, 7, 8-Tetrabromo-2, 3-dihydro-1, 4-phthalazinedione was synthesized using 90 min reflux of the mixture of tetrabromo phthalic anhydride (0.0043 mol) and hydrazine hydrate (0.063 mol) in ethanol (2 mL) at 80 °C. The precipitate was a yellow one which was washed with water and purified by stirring in hot ethanol.
Polyurea synthesis
Polyureas, based on reactions between 2, 3-dihydro-1, 4-phthalazinedione (0.0067 mol) and three different diisocyanates (0.0067) in dry N, N-dimethylacetamide (0.5 mL) as the solvent were synthesized in three mixing steps at different temperatures and periods (Table 1). The third step begun as the mixture viscosity raised. Adjusting the mixture viscosity, some drops of solvent (N, N-dimethylacetamide) were added to the mixture. The obtained white polymers were precipitated in methanol at the end of the reaction. The schematic of these synthesizes is illustrated in Scheme 1.
Synthesizing polymer (D), Toluylene diisocyanate (0.00022 mol), and the exact molarity of 5, 6, 7, 8-Tetrabromo-2, 3-Dihydro-1, 4-phthalazinedione were mixed in 0.5 mL solvent of dry N,N-dimethylacetamide for about 24 h at 25 °C. Then the temperature was raised to 40 °C for another 24 h of stirring. Finally, with increasing the viscosity, the temperature was raised to 85 °C. The pale-yellow precipitants in methanol were collected. Scheme 2 illustrates the schematic of reactions involved in Polymer (D) synthesis.
Result and Discussion
Structure validation of polyureas
IR and elemental analysis were done to validate the polyurea's chemical structure. IR spectra of four synthesized polyureas are shown in Figure 1. Table 2 describes the characteristic peaks in IR spectra.
As expected, the C=C symmetric and asymmetric stretching, C-H stretching in the aromatic ring, carbonyl (C=O), and N-H stretching belonging to 2, 3-dihydro-1, 4-phthalazinedione commonly can be seen in all products. There are methylene (-CH2) bending peaks in IR spectrums of Polyureas (B) and (C). Moreover, the stretching peak of C-Br has only presented in the IR peak of polyurea (D) [20‒22].
The quantities of C, H, and N in the expected chemical structures of the four polyureas (A) to (D), were calculated theoretically and analyzed experimentally. The significant affinity of calculated quantities and experimented ones in Table 3 represents the accuracy of the proposed chemical structures for all four synthesized polyureas.
Table 1. Mixing conditions in the procedure of synthesizing of polymers A, B, and C
|
Polyurea |
Diisocyanates |
Step 1 |
Step 2 |
Step 3 |
| (A) | Toluylene diisocyanate |
Mixing at 25 °C for 3 hours |
stirring at 50 °C for 12 hours |
kept on stirring at 85 °C for 24 hours |
| (B) | Isophorone diisocyanate |
Mixing at 25 °C for 48 hours |
stirring at 60 °C for 5 hours |
kept on stirring at 80 °C for 20 hours |
| (C) | Hexamethylene diisocyanate |
Mixing at 25 °C for 20 hours |
stirring at 60 °C for 24 hours |
kept on stirring at 85 °C for 24 hours |
.jpg)
Scheme 1. The schematic of the polymer A, B, and C synthesizes
.jpg)
Scheme 2. The schematic of reactions involved in polymer D synthesis
.jpg)
Figure 1. IR spectra of four synthesized polyureas of a) A, b) B, c) C and d) D
Table 2. Details of IR peaks in A, B, C, and D polyurea IR spectra
.jpg)
Table 3. Results of elemental analysis of A, B, C, and D polyureas
|
Polyurea |
|
C (%) |
H (%) |
N (%) |
|
(A) |
Exp: |
60.53 |
4.56 |
17.23 |
|
Cal: |
60.714 |
3.596 |
16.659 |
|
|
(B) |
Exp: |
61.67 |
5.20 |
15.22 |
|
Cal: |
62.486 |
6.292 |
14.573 |
|
|
(C) |
Exp: |
58.56 |
6.32 |
17.14 |
|
Cal: |
58.174 |
5.492 |
16.96 |
|
|
(D) |
Exp: |
32.60 |
2.03 |
8.71 |
|
Cal: |
31.322 |
1.236 |
8.594 |
Characteristic study of synthesized polyureas:
Results of melting temperatures, intrinsic viscosities , the viscosity average molecular weights, and the degree of polymerization of polyureas are presented in Table 4.
Based on these data, the melting points of the synthesized polyureas are ordered as C, D, B, and A, respectively. The higher melting temperature of polyurea D than the B and A one’s, despite its lower degree of polymerization (DP: versus ) can be attributed to the excessive polarity in the structure of polyurea D caused by electronegative atoms of Br.
In the case of polyureas of A and B with almost the same degree of polymerization, consequently same chain entanglements, polyurea A was melted at a higher temperature than B. Using aromatic and cyclic structure in the backbone of polyurea A, led to a stiffer backbone which boosts the crystallinity and an increase in the melting temperature (i.e., Stiffer polymers represent higher melting temperatures [23]). And lastly, the highest melting temperature of polyurea C can be due to its higher degree of polymerization.
Figure 2 represents the TGA, and derivative thermogravimetry (DTG) of polyureas. 2 stages of decomposition peaks were observed in DTG curves of polyureas (A) to (C). The weight losses at 292-295 °C that are common in polyureas (A) to (C) in DTG curves, can be attributed to 2, 3-dihydro-1, 4-phthalazinedione degradation. The weight loss at 180, 263, and 350 °C in the DTG curves of polyurea (A) to (C) arose from three different diisocyanates containing aromatic, cyclic aliphatic, and linear aliphatic nature of R groups, respectively, in their structure. As expected, the weight losses related to the polyurea with more aliphatic groups were higher than the aromatic ones.
In the case of polyurea (D) with four electronegative atoms of Br with non-bonded electrons on the 5, 6, 7, 8-Tetrabromo-2, 3-dihydro-1, 4-phthalazinedione groups, have led to more resonance structures and more stable structures with just one weight loss peak at 285 °C.
Solubility parameters of any polymer guide the solvent selection, depending on its chemical structure, can be estimated by group molar attraction constants [24⎼26]. The solubility parameters of the newly synthesized polyureas were calculated using the Equation 1 and reported in Table 5.
![]()
Where is the solubility parameter, is the polymer density, is the the molar attraction constants and is the molecular weight of monomer.
Different solvents were investigated for new polyureas. As seen in Table 6, the majority of solvents cannot be considered suitable solvents for prepared polyureas, which is following the high calculated quantities of solubility parameters (Table 5).
Table 4. Melting temperatures, viscosities, molecular weights, and degree of polymerization of synthesized polyureas
.jpg)
.jpg)
.jpg)
Figure 2. a) TGA and b) derivation of TGA of polyureas of A, B, C, and D
Table 5. Calculated solubility parameters of polyureas
|
Polyurea |
M0 (g/mol) |
Σ G (cal. Cm3)0.5 |
δ (cal. cm3)0.5 |
|
(A) |
336 |
10158 |
37.79 |
|
(B) |
384 |
11594 |
43.132 |
|
(C) |
330 |
10006 |
37.22 |
|
(D) |
651.6 |
12714 |
47.29 |
Σ G calculated based on Hoy constants [27]
Table 6. Solubility study of newly synthesized polyureas in several solvents
|
|
Polyurea |
||||
|
Solvent |
δ (cal. cm3)0.5 |
(A) |
(B) |
(C) |
(D) |
|
Eethyl acetate |
7.9 |
* | * | * | * |
|
Toluene |
8.9 |
* | * | * | * |
|
Tetrahydrofuran |
9.52 |
* | * | * | * |
|
Dichloromethane |
9.93 |
* | * | * | * |
|
Acetonitrile |
11.9 |
* | * | * | * |
|
Ethanol |
12.92 |
* | * | * | * |
|
Methanol |
14.5 |
* | * | * | * |
|
Dimethylacetamide |
22.1 |
* | * | * | * |
|
water |
23.5 |
* | * | * | * |
|
Dimethyl formamide |
24.8 |
* | * | * | * |
|
Dimethyl sulfoxide |
26.6 |
P |
P |
P |
P |
*: Insoluble
P: Partially soluble
Conclusions
In this research study, four polyureas derived from aliphatic and aromatic diisocyanates were synthesized. The chemical structure of these polyureas was validated using IR and elemental analysis. Molecular weights, rheological, thermal, and solubility properties of these new polyureas were calculated theoretically and evaluated experimentally. According to these assessments, the new polyureas had an almost stiff structure with good thermal stability and were insoluble in different solvents.
Disclosure Statement
The authors reported no potential conflict of interest.
Orcid
Shahrzad Rahmani 0000-0001-9278-3118
How to cite this manuscript: Sanaz Mohammadzadeh KoumLeh, Shahrzad Rahmani*, Hosein Nasr-Isfahani. Synthesis, characterization and comparative study of aromatic polyureas based on 2, 3-dihydro-1, 4-phthalazinedione with various aromatic and aliphatic diisocyanates. Asian Journal of Green Chemistry, 6(2) 2022, 145-154. DOI: 10.22034/ajgc.2022.2.4